Novocart 3D is a new investigational product in the United States but has been widely used in Europe since 2003 to treat patients suffering from damaged cartilage. Patients are being asked to partake in this clinical trial assessing the pain and physical function of the knee after one of two possible surgical treatments. Participants will be randomly assigned to one of two surgical patients in a ration of 2:1 (patient is more likely to receive Novocart 3D than Microfracture).
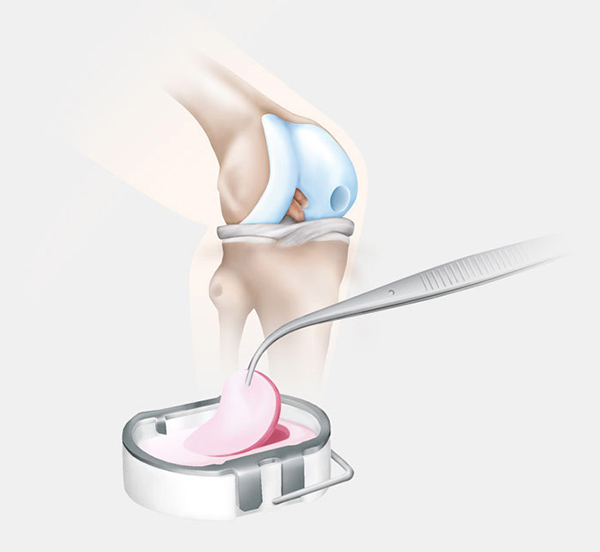
One surgical procedure in the study utilizes the Novocart 3D autologous chondrocyte implant system. Patients in this arm of the study will be receiving an implant populated with their own cartilage cells. This procedure takes place over the course of two surgeries:
- Cartilage Extraction – Samples of the patients knee cartilage are arthroscopically removed and sent to a lab where the cells are cultivated;
- Transplantation (approx. 3 weeks later) – The damaged cartilage is removed and the implant is shaped to fit the defect and fixed.
The other procedure, Microfracture, requires creating several small holes in the bone within the cartilage defect. These holes cause bleeding within the defect, resulting in a clot and eventually the clot allows for new cells to form “repair cartilage” that fills the defect. Microfracture is one of the current gold standard treatment options for cartilage defects.
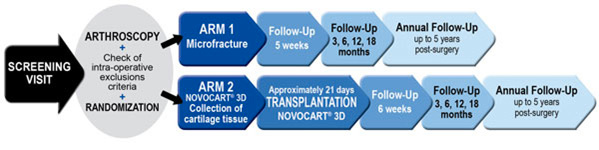
Clinical Trial Schedule:
To know more about Novocart 3D clinical trial, please click here.